What is Buprenorphine?
Buprenorphine Brand names:
Suboxone®1, Subutex®2, Zubsolv®3, Bunavail®4, Probuphine®5
Buprenorphine
Buprenorphine is an opioid medication used to treat opioid addiction in the privacy of a physician's office. It can be dispensed for take-home use, by prescription. This, in addition to the pharmacological and safety profile, makes it an attractive treatment for patients addicted to opioids.
At the correct dose, buprenorphine suppresses cravings and withdrawal symptoms and blocks the effects of other opioids.
Buprenorphine is not new. It was first patented in 1969 and has been used in the U.S. to treat pain and in Europe to treat pain and opioid addiction for over 20 years, and is a semi-synthetic opioid and is a partial agonist.
- Opioid Agonists are drugs that cause an opioid effect like heroin, oxycodone, hydrocodone, and methadone.
- Opioid Antagonists are drugs that block and reverse the effects of agonist drugs. Narcan® is an antagonist and is used to reverse heroin overdoses.
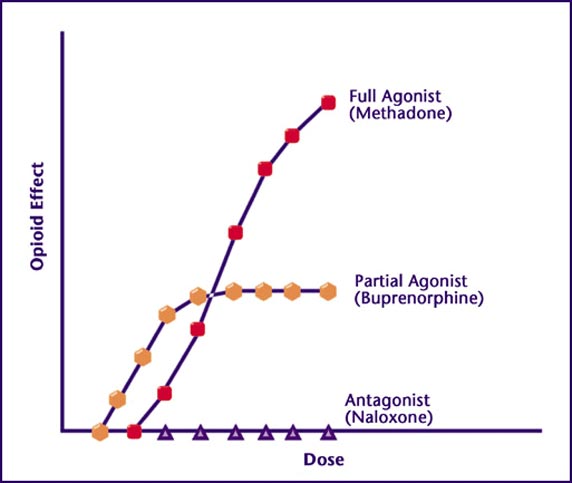
Buprenorphine can act as both an agonist and antagonist. It attaches to the opioid receptors but only activates them partially, enough to suppress withdrawal and cravings, but not enough to cause extreme euphoria in opioid-tolerant patients. When all available receptors are occupied with buprenorphine, no additional opioid effect is produced by taking more. This is called the 'ceiling effect'. The antagonist property of the medication expels, replaces and blocks other opioids from the opioid receptor sites. Therefore, if the patient decides to misuse opioid drugs after taking buprenorphine, the effects can be blocked, depending on dosage. Alternately, if taken too soon after other opioids, by an opioid-physically dependent patient, the buprenorphine can precipitate withdrawal. The ceiling effect, blocking ability, and possibility of precipitating withdrawal, contribute to buprenorphine having a favorable safety profile and helps lower the risk of overdose and misuse.5
Buprenorphine (Bup) and
Buprenorphine / Naloxone (bup/nx) combination
In October 2002 the FDA approved the first two prescription bup medications for the treatment of opioid addiction; Subutex® (bup) and Suboxone® (bup/nx). Since 2009 the FDA approved generic bup and bup/nx sublingual tablets, the brand-name bup/nx sublingual tablet called Zubsolv®, a buccal film bup/nx product called Bunavail®, and a 6-month buprenorphine implant called Probuphine®. Both Suboxone and Subutex tablets were discontinued and replaced with Suboxone Film® which is a bup/nx sublingual film.
UPDATE:
on 6/14/2018 the FDA approved the first generic versions of Suboxone FILM.
The only reason for the addition of naloxone is to discourage misuse by injection. If the bup/nx combination is injected, the naloxone can help cause immediate withdrawal symptoms in opioid-physically dependent people. However, naloxone is poorly absorbed sublingually. Therefore, when taken as directed, very little naloxone enters the blood. Normally, patients are unaffected by the presence of it, and it is considered clinically insignificant. Buprenorphine itself can cause withdrawal in physically dependent people misusing full agonist opioids. Naloxone may only slightly attenuate the effects of buprenorphine if misused by injection.
Isn't buprenorphine just switching one addiction for another?
No, because once stabilized in bup or bup/nx treatment, the compulsive behavior, the loss of control of drug use, the constant cravings, and all of the other hallmarks of addiction vanish. When all signs and symptoms of the disease of addiction vanish, we call that remission, not switching addictions. Remember, it is the uncontrollable compulsive behavior that we're looking to stop, because that's what's destructive, not taking a medication. Switching addictions, would mean you have lost control of your buprenorphine use, use it compulsively, and continually crave your buprenorphine. Generally none of this is experienced by typical buprenorphine patients, although it is possible and should be watched for. Instead most buprenorphine patients have complete control of their buprenorphine use, don't use it compulsively and don't crave it.
Yes, a physical dependence to opioids remains and is maintained by the buprenorphine, but physical dependence is not addiction, does not destroy lives, and is relatively easy to reverse with a slow taper off of the medication at the appropriate time.
With all the drug war propaganda we've been exposed to it's easy to mistake the problem as a drug problem when really it is the addiction that is destructive. Don't make the mistake of dismissing evidence-based treatments because you think you have a drug problem and taking drugs for a drug problem seems counter productive. It's not a drug problem, it's an addiction problem and if a drug is needed to stop the addiction then so be it.
- More frequently asked questions here -
How Buprenorphine works
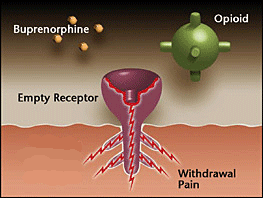
Opioid receptor is empty – As someone becomes tolerant to opioids, they become less sensitive and require more opioids to produce the same effect. Whenever there is an insufficient amount of opioid receptors activated, the patient feels discomfort. This happens in withdrawal.
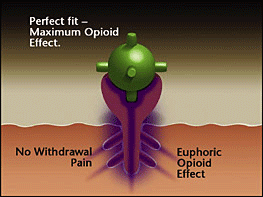
Opioid receptor satisfied with a full agonist opioid - The strong opioid effect of heroin and painkillers can cause euphoria and stop the withdrawal for a period of time (4-24 hours). The brain begins to crave opioids, sometimes to the point of an uncontrollable compulsion (addiction), and the cycle repeats and escalates.
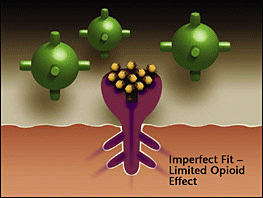
Opioids replaced and blocked by bup - Buprenorphine competes with the full agonist opioids for the receptor. Since bup has a higher affinity (stronger binding ability than other opioids) it expels existing opioids and blocks others from attaching. As a partial agonist, the bup has a limited opioid effect, enough to stop withdrawal but not enough to cause intense euphoria.
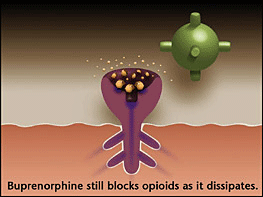
Over time (24-72 hours) bup dissipates - but still creates a limited opioid effect (enough to prevent withdrawal) and continues to block other opioids from attaching to the opioid receptors. Since bup is long acting, patients remain out of withdrawal between doses.
References:
- Suboxone tablets FDA approved in Oct. 2002. Tablets discontinued in the US in 2011 in favor of a film version.
- Subutex (bup) brand tablets discontinued in the US in 2009. Generics available.
- Zubslov, brand name bup/nx tablet better bio-availability, lower dose, with better taste. - savings card - website
- Bunavail DISCONTINUED IN 2020, Bup/Nx FDA approved 6-6-2014. buccal film, better bio-availability, lower dose, less side effects. - savings card - website
- Probuphine Bup 6 month implant, FDA approved 5/26/2016 - available now
- National Alliance of Advocates for Buprenorphine Treatment- www.NAABT.org